-
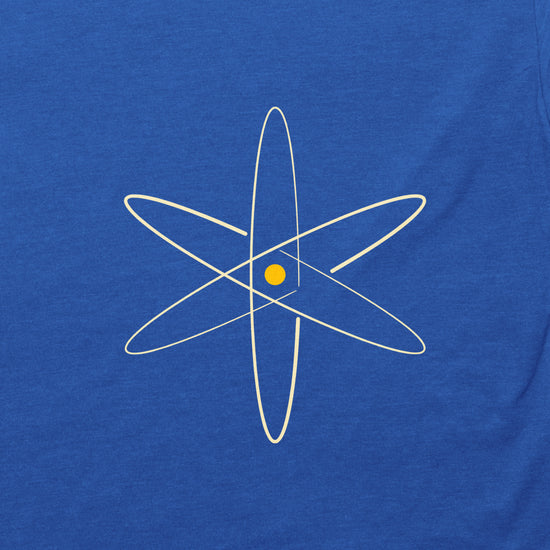
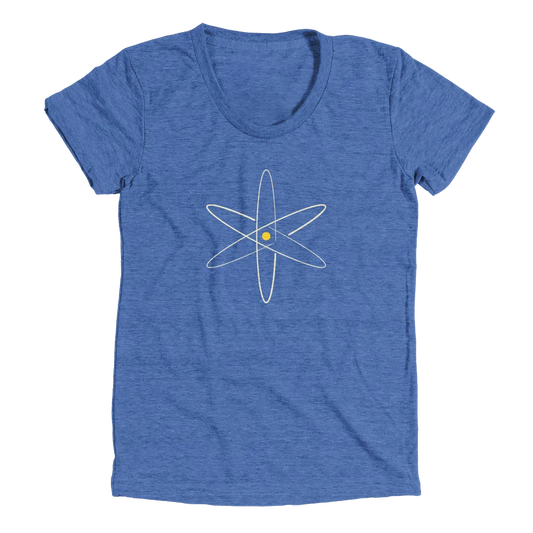
Nuclear Model
The Nuclear Model of the Atom, brought to us by Ernest Rutherford, was the first to place the positive charge at the nucleus of the atom.
-

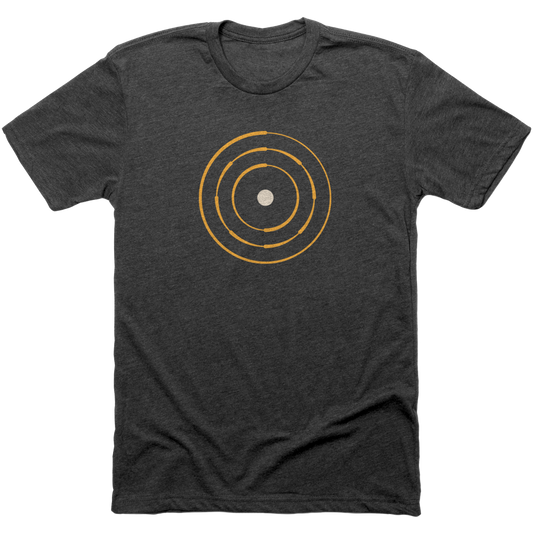
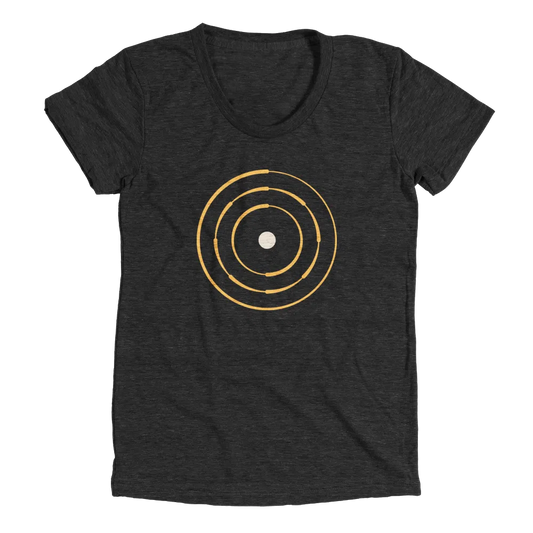
Planetary Model
The Planetary model of the atom pioneered by Niels Bohr. This model stated the electrons revolved around the nucleus in fixed orbits.
-

Quantum Model
The quantum model of the atom was developed by Erwin Schrodinger. It showed that electrons don't move, but instead exist in a cloud of probable locations.
-
Quantum Model
Regular price From $32.00 USDRegular priceUnit price per -
Nuclear Model
Regular price From $32.00 USDRegular priceUnit price per -
Planetary Model
Regular price From $32.00 USDRegular priceUnit price per -
Quantum Model - Women's Cut
Regular price From $32.00 USDRegular priceUnit price per -
Nuclear Model - Women's Cut
Regular price From $32.00 USDRegular priceUnit price per -
Quantum Model Long Sleeve
Regular price From $35.00 USDRegular priceUnit price per -
Nuclear Model Long Sleeve
Regular price From $35.00 USDRegular priceUnit price per -
Planetary Model - Women's Cut
Regular price From $32.00 USDRegular priceUnit price per -
Nuclear Model Hoodie
Regular price From $49.00 USDRegular priceUnit price per -
Planetary Model Long Sleeve
Regular price From $35.00 USDRegular priceUnit price per -
Nuclear Model Sweatshirt
Regular price From $48.00 USDRegular priceUnit price per -
LHC Sweatshirt
Regular price From $48.00 USDRegular priceUnit price per